What You Need to Know About the New FDA Vaccine Rules
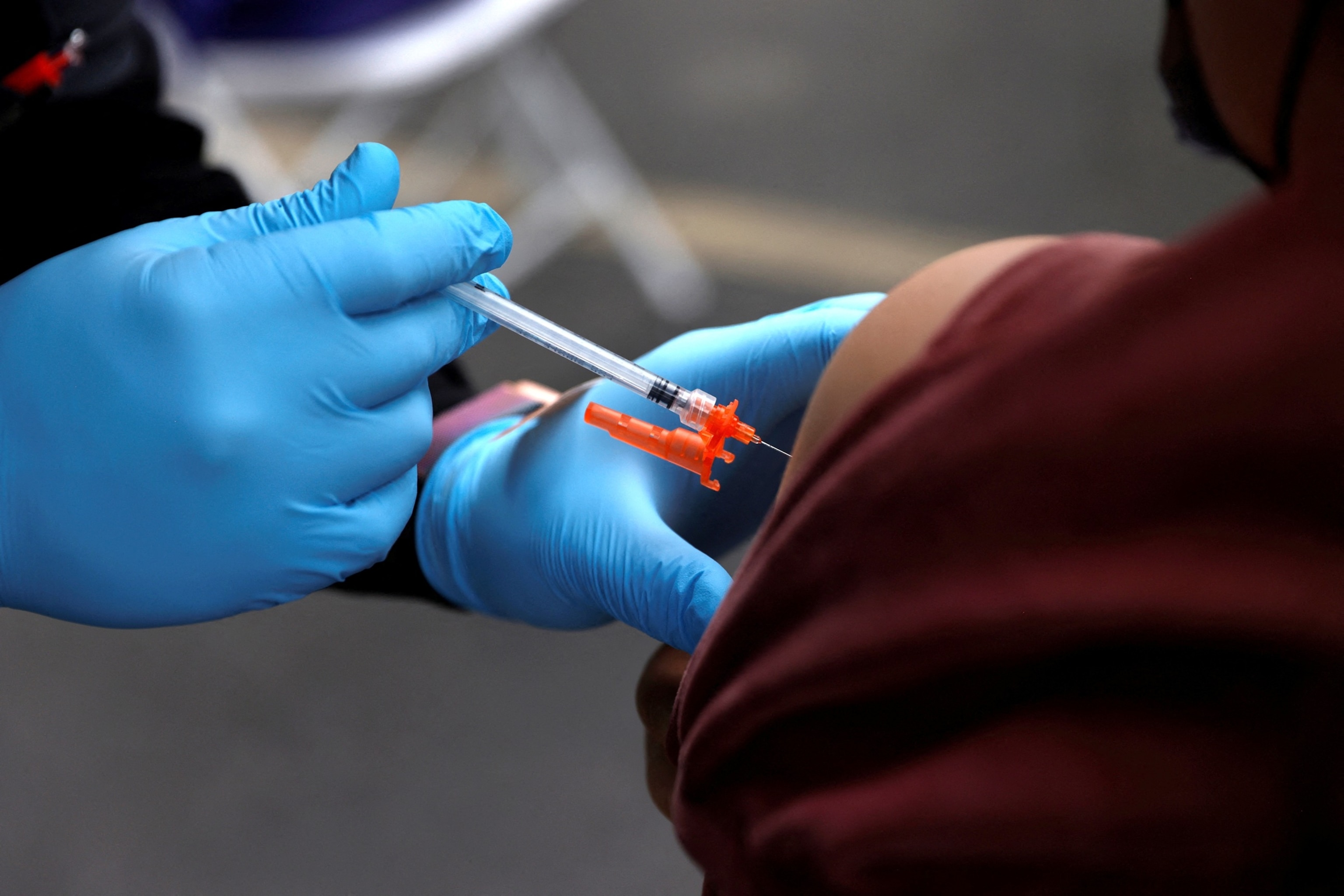
Changes in FDA Guidelines Impact Vaccine Access for Younger Americans
In August, the U.S. Food and Drug Administration (FDA) updated its approval of the latest COVID-19 vaccines, limiting their availability to individuals over 65 or those with underlying health conditions that increase the risk of severe illness. This decision has sparked concerns about access for millions of Americans who fall outside these categories, including younger, healthier individuals.
According to pharmacists, insurance groups, and trade organizations, younger adults may face additional challenges in obtaining the vaccine as the policy changes take effect. Instead of simply visiting a pharmacy, they may need to consult a doctor, which could complicate the process. Additionally, insurance coverage for these individuals remains unclear for now.
The shift in policy also affects older, high-risk individuals, as some pharmacies are still requiring prescriptions for them to receive the vaccine. This confusion may lead to delays or difficulties in accessing the shots during the upcoming respiratory virus season.
Despite these concerns, officials from the Trump administration have emphasized that all adults can still choose to get vaccinated. FDA Commissioner Marty Makary stated on X that "we are not limiting availability to anyone." Similarly, White House press secretary Karoline Leavitt affirmed this stance, emphasizing individual choice and the commitment of the president and Health and Human Services Secretary Robert F. Kennedy Jr. to ensure vaccine access.
Experts interviewed by ABC News note that while the policy is technically accurate—since doctors can still prescribe the vaccine off-label for non-eligible individuals—the practical implications may be more complex. The added step of visiting a doctor, combined with potential hesitancy among healthcare providers to go against federal guidelines, could create significant barriers for those seeking the vaccine.
This situation highlights the growing challenge of vaccine uptake, particularly among younger, healthier populations. For years, public health officials have encouraged annual vaccination, similar to the flu shot. However, under the previous administration, the focus shifted toward emphasizing the benefits of vaccination for older, high-risk individuals, with less emphasis on young, healthy people.
Recent data from the Centers for Disease Control and Prevention (CDC) shows that only 23% of adults and 13% of children received the updated vaccine last season. In contrast, 49% of adults aged 75 and older had received the latest vaccine as of December 2024.
State Laws Vary in Vaccine Access
The rules surrounding vaccine access differ across states. In some states, pharmacists are restricted to administering vaccines only to those who meet FDA and CDC guidelines. This means that in certain areas, pharmacies may no longer offer the vaccine to individuals who do not qualify under the new FDA approvals due to liability concerns.
States like Massachusetts, New Jersey, Pennsylvania, and New Mexico have recently adjusted their laws to allow pharmacists to continue offering the vaccine despite the FDA's changes. However, the patchwork of state regulations makes it difficult for patients to know where they can access the vaccine.
CVS, the largest pharmacy chain in the country, continues to offer the vaccine in 41 states for those who meet the FDA’s updated criteria. However, in nine states plus Washington, D.C., individuals will need a prescription to receive the shot, even if they qualify under the new guidelines. These states include Arizona, Florida, Georgia, Louisiana, Maine, North Carolina, Utah, Oregon, and West Virginia.
Walgreens has stated it will provide the vaccine where legally permitted but has not specified which states will follow which rules.
Insurance Coverage and Uncertainty
Insurance coverage for the vaccine remains uncertain, particularly for younger, healthy adults. Private insurers have indicated they may cover the cost of the vaccine for anyone receiving it, but they are awaiting guidance from the CDC’s advisory panel, which is set to meet in late September. This period of uncertainty could leave many without clear options just as the fall and winter seasons approach, when cases may rise again.
The meeting of the advisory panel has also been called into question due to recent turmoil at the CDC, including the departure of several officials and a public dispute between Kennedy and the former CDC director, Susan Monarez. Republican Sen. Bill Cassidy has called for the meeting to be postponed.
Insurance providers are monitoring the situation closely. Tina Stow, spokesperson for America’s Health Insurance Plans, noted that plans will make coverage decisions based on science and medical evidence. However, the unpredictability of the new advisory panel, composed of members handpicked by Kennedy, adds to the uncertainty.
Challenges for Children and Government-Funded Programs
The impact of the FDA’s decision extends to healthy children under 5, who are no longer approved for any COVID vaccines. Previously, they were authorized to receive the Pfizer vaccine, which was available to children as young as 6 months. This change raises concerns about access for this vulnerable group.
Government-funded programs such as Medicare, Medicaid, and the Vaccines For Children program are tied to CDC recommendations, making the future of vaccine access for low-income families unclear. As the landscape of vaccine policy continues to evolve, the challenges for younger and healthier individuals remain significant.
Post a Comment